Airway management seems like the current flavour. Actually it’s sort of always the flavour. Finally, Dr Alan Garner has something to say about something that isn’t about first pass success – checklists.
At the risk of treading into an area likely to stir up as much passion as first pass success, it’s time to talk about checklists. There’s a new publication out there touching on standardisation and the use of checklists among teams providing prehospital drug assisted intubation that has just been published. You can find it here, although it is not open access unfortunately.
The authors surveyed services that they could identify providing prehospital emergency anaesthesia in the UK and sent them a questionnaire. 43 services participated. There was a spread of helicopter and road-based services in addition to three ED-based teams representing 75% of UK services. That’s a reasonable sample.
The issue that particularly grabbed my interest was the use of checklists. Most reported services used checklists, particularly the busier ones. Many services have a longer checklist they use for drugs assisted intubations and another shorter one they use for crash intubations. But unlike any paper I have seen previously this study gives a lot of detail about the checklists themselves, things like the number of items on the checklist, the wording and formatting.
The thing that caught my eye was the length and complexity of the checklists. To directly quote the study:
“On average, standard checklists contained 169 (range: 52–286) words and 41 (range: 28–70) individual checks.”
That caught my eye because the service I’m working in is a massive outlier when it comes to checklists. Out standard prehospital intubation checklist has 13 items when counted using the methodology from this paper.
This is less than half the items on the shortest checklist reported in the study which had 28 items. That’s startling enough to have another look.
Let’s Look Then…

First thing that is worth saying is that this is the checklist utilised by our rapid response helicopter service in Sydney. This service does just one thing which is prehospital trauma response within a 30 minute flight time of our Sydney base. No inter-facility transfers which have a quite different workflow. In those longer haul operations we use a longer checklist which is this one:
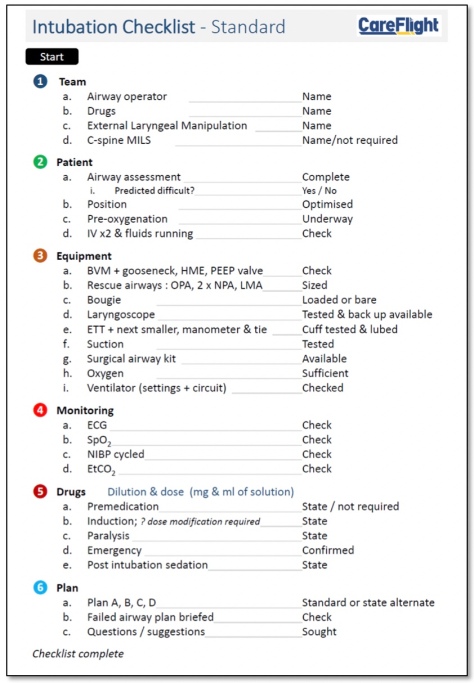
This checklist has 40 items which places it in the middle of the pack compared with this UK study. We use this checklist in our Northern Territory and international jet operations where intubation is a less common event for the teams. Many of the referring sites are small and sometimes have no staff with advanced airway skills. Plenty have no plumbed oxygen systems, relying on bottled gas. In our international jet operations the staff at the referring hospital may speak limited or no English and getting assistance or additional equipment can be very challenging. We therefore take nothing for granted and check everything. This appears to be comparable to the reported checklists in the study.
P is for Prehospital
But the study is about prehospital anaesthesia specifically. Many of the reported services, particularly the HEMS services, are like our Sydney service and conduct only prehospital operations. Our standard prehospital intubation checklist in Sydney is more equivalent to the “crash induction” checklist mentioned in the study both in number of items checked and word count but we use it for all intubations whether time pressured or not.
So why is our standard prehospital checklist such a dramatic outlier and why do we only have a short checklist that we use all the time? Did we sit down, follow the KonMari method and ask if every individual item on the checklist gave us joy? Well, no.
Before we look at this I should say that I’m pretty happy that our success and complication rates are very good compared with the published literature. You can see some of this in previous posts about how we measure quality in intubation practice here and here. So being bad at it and accepting lots of complications is not the explanation.
When your thing does the opposite of what you want it to do
To explain this we need to have a look at how checklists can sometimes hinder what we do. As checklists have been increasingly adopted in medicine and other safety critical industries the potential problems associated with their use are becoming clearer. Some of these are cultural – do the teams actually use the checklists in the way they were intended? Do they use them at all despite an SOP mandating them? Some of the resistance to checklists has been perception that they are just a “tick and flick” exercise for audit purposes but don’t really improve patient safety. That they slow things down and get in the way of patient care. Or that the items on the checklist are not really relevant or the list is too long and onerous. A level of checklist fatigue can result with personnel hurrying through them without really paying attention or omitting them altogether.
At this point I would seriously recommend having a listen to Martin Bromiley, a pilot whose wife died due to human factors issues during a routine operation. He discusses what checklists are and are not.
To mitigate these factors checklists need to be short, and the list needs to have only items that both can be omitted by oversight but at the same time are critical to safety. But I don’t think some of the items on the lists in the study meet these criteria or the issue they attempting to address can be managed in another way by re-engineering the process.
Pursuing Simplicity
To illustrate what I mean 100% of standard checklists in the study had an item to ensure that an IV line had been placed and was patent. But it is impossible to proceed with a drug assisted intubation without functioning venous access (whether IV or IO). If you attempt to proceed without having checked this item you will rapidly come to halt anyway.
In other words it is not possible to omit this item whether you check it or not. So why check it? You are just wasting time.
An example of engineering out a source of error is the oxygen supply. When I worked in the UK myself years ago we did not routinely carry oxygen to the scene. You had no control over how full the bottle that came with the ground ambulance was and you needed to check every time. And the bottles that were available were only 400L which did not last all that long on a mask on high flow in any case.
Our approach is to carry our own 600L O2 bottle to every case, and use it for the intubation process every time. We checked it either in the morning checks, or after the prior case so we know it is full. So we don’t check it again.
This is another part of shortening the checklist. If you can check it before the phone goes off do so. Our checklist is really focused on the factors that we could not do before we met the patient because we did not know who the patient was and what their issues were.
Our checklist aims at optimising the process for that specific patient in terms of plan, positioning, specific drug selection and getting out the right size equipment. But everything that we could check before hand was already done and we don’t check it again.
You don’t make sure every nut on the helicopter is properly torqued before you depart on a mission because that has already been done, and this should be no different. Most of the equipment items mentioned in table 3 of the study fall into this category. We check our laryngoscope and ventilator every morning and we don’t do it again on the scene. We have had no failures of either over the past 13 years.
The only other things we do are check that we have the suction out and the monitoring on – simply because it is possible to proceed without these being in place and both are critical for patient safety. These are the items that a checklist was really designed for.
Having said this we always carry a copy of the longer checklist that we use in our inter-facility operations. If we are tasked to another case before we can properly redo our checks, or either of the prehospital team members is just not happy for any reason the team reverts to the full check list although in practice this occurs very rarely.
Getting the Team Onboard
I think it is basic human behaviour that compliance with a process will be better when team members can see that it is just what is required without unnecessary steps. That the really critical components are captured which protects both the patient and themselves.
But I think that they also appreciate a carefully designed process that has removed the requirement for additional checks by engineering out the possibility of error in the first place wherever possible. If the whole team is actively involved in process redesign through identifying and eliminating opportunities for error they own the resulting shorter checklist. They follow it because they know if the item is still on the checklist then it both matters and we could not find an alternative to checking it on the scene.
So in the end we have very high success and low complication rates but with a very brief checklist. But maybe this story is more about empowered teams, and the never ending quest for quality.
And the challenge is always there: does the checklist provide what patients and crew need? And is every item there useful, or could you have sorted it earlier so you just do the vital bits to get the job done in the moment?
Notes:
Feedback is great because we don’t get better without hearing from clever people. So drop a comment. You might be the person who shows us something we could improve.
That paper again is this one:







![What was the big picture again? [via Jarod Carruthers on flickr under CC 2.0 and unaltered]](https://careflightcollective.files.wordpress.com/2015/07/trees-copy.jpg?w=474&h=143)

![Here is a visual metaphor for the next segue [via www.worldette.com]](https://careflightcollective.files.wordpress.com/2015/05/viaduct-copy.jpg?w=474&h=314)