Continuing the series of sharing Carebundles, Alan Garner moves on to go through the stuff to include in multiple blunt trauma.
OK, part 2 in our Carebundle series. This time we will take a look at our multiple blunt trauma bundle. This excludes isolated head injury which we dealt with in the previous post. Why that order you may ask? Our Sydney service started life as a trial evaluating the management of severe head injury so TBI is front if mind for us. It is also more straightforward as there are not the competing priorities that occur in multiple trauma. And in the end we don’t just want survivors but neurologically intact survivors so starting with TBI and brain resuscitation makes sense. The multiple blunt trauma bundle has conditional targets that are modified by the presence or absence of brain injury acknowledging that brain resuscitation is our major goal.
So multiple blunt trauma is next. This has many bits of intrigue to it. It is multiple. We’re moving into the bits of the body where the pathology can be buried in the large splodgy bit in the middle. The diagnostic stuff can be pretty challenging at the side of the road. Oh, and because it’s multiple there’s always that threat of a new competitor emerging in the pathophysiology parade.
We won’t touch on penetrating trauma, burns and immersion all of which have their own bundles of joy for another time.
The Common Touch
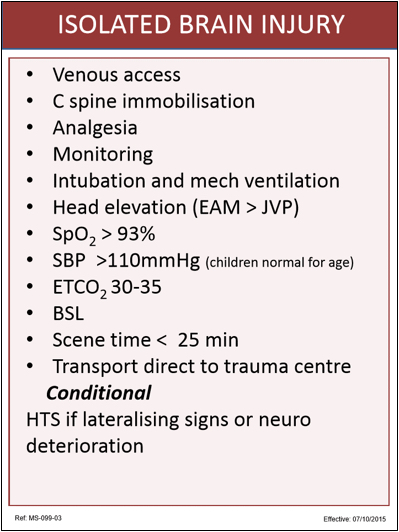
All of the mandatory items overlap with the TBI bundle so we won’t waste any time on them here:
- Venous access – yes we reckon that still makes sense.
- Analgesia – opioids/ketamine – yes we’re really trying to stress that analgesia is a vital component of care, pretty much every time.
- Monitoring: SpO2, NIBP, ECG
- Spine immobilisation – note we’re just sticking with immobilisation.
- SpO2 > 93% by ED arrival
- Scene time < 25 min – again, this isn’t always possible which is part of why Carebundles provide guidance but need clinician judgment on each job. What we’re aiming for is a background enthusiasm for keeping momentum throughout the time we’re looking after patients so we can get them to the hospital with all those eager people waiting.
- Transport direct to trauma centre – this would be the house for the eager people.
The conditional items however vary from the TBI bundle and we will now go through these.
Checking the Terms and Conditions
Long bone fractures splinted
There is no evidence I am aware of that this changes outcome but it is standard ATLS teaching and makes pain control easier. We carry lots of excellent drugs and the Carebundle makes a point of mentioning them but everything is easier if you manage the physical elements contributing to the painful situation. Really this is the original multimodal analgesia. It’s just that one of the modes is “physical things that stop hurting things from exercising a right to freedom of movement”.
Massive external haemorrhage controlled
There is strong cohort level data that this saves lives, although more so in the penetrating trauma context where it is more common. Certainly data from recent conflicts supports this as a primary aim of prehospital care. So we’re carrying tourniquets, dressings, chitosan gauze and granules (though the latter are more for penetrating wounds).

TXA if episode of SBP < 90mmHg, or below normal for age
CRASH 2 inclusion criteria were felt to be a little vague to include in our bundle. After all the inclusion criteria in this study was any trauma patient who was at risk of haemorrhage. To make the bundle we felt the item needed to identify the cases where TXA really should have been given because the risk of life threatening haemorrhage is so high. There is some evidence that just a single episode of documented hypotension is enough to identify a group of very high risk patients so we adopted this as our criteria. As another mental trigger point, some of our team have expressed a process when they consider packed cell transfusion – “If I’m reaching for blood, I should reach for that drug.”
If shocked, SBP at ED arrival (refer fluid guideline)
- No head injury: palpable central pulses/obeying command
- With head injury: Palpable peripheral pulses, or SBP > 90mmHg / lower limit of normal for age
In setting our blood pressure targets we differentiated between those with and without head injuries. Without a head injury permissive hypotension is our strategy. With a head injury we adopted the lowest level identified in the Brain Trauma Foundation Guidelines i.e. SBP of 90mmHg as our target. This is lower than our target for isolated severe TBI where our target is a MAP of 90mmHg or SBP of 110mmHg (see the TBI bundle post for further details). That last modification is obviously for paediatric patients where the guidelines are a little harder to attach specific numbers to.
If GCS < 9:
- Intubation and mechanical ventilation
- EAM above JVP (head elevation)
- ETCO2:
- 30-35mmHg if no chest trauma/shock
- 25-30mmHg if chest trauma/shock present
This is similar to our isolated severe TBI bundle but we finesse our etCO2 targets in the presence of other injuries that might affect the gradient between arterial and alveolar levels. There is some evidence that adopting a lower prehospital etCO2 target in patients with chest trauma and/or shock is reasonable as these patients have predictably higher gradients. My own personal experience is that in patients who have both chest trauma and shock the target needs to be even lower. I have achieved an etCO2 by ED arrival in the mid-twenties in patients where both these factors are present only to find the first blood gas reveals an arterial level in the 50s. I would certainly be interested in hearing other people’s experience on this one. Of course in our rapid response urban trauma work we don’t carry a POC blood gas analyser like we do in our interfacility transport operations. Actually measuring the arterial CO2 would be ideal but we don’t think this is practical for both time and weight reasons in our urban response service.
Thoracic decompression if hypoxic/shocked & clinical or US suspicion of pneumothorax
I don’t think this one is rocket science. Even if we know a pneumothorax is present on ultrasound we usually leave it alone if they are not compromised. If compromise is present however then we expect it to be decompressed.
If GCS <13, BSL documented
All patients with an altered level of consciousness get their blood glucose documented.
Pelvic binder if shock and:
- possible AP compression / Vertical Shear injury or signs of pelvic #
We don’t expect pelvic binders to be placed prophylactically. There is no evidence to support such a practice. We do however think that binders are helpful on AP compression and possibly vertical shear type injuries and the patient is shocked.
So that is it for our multiple blunt trauma bundle. It’s what we came up with on a review of the evidence but we’re always open to clever thoughts from others. If you have comments or suggestions we would love to hear from you.
And next time we return to the Carebundles it might just be time to get to the pointy end of penetrating trauma.
Notes:
As always, we’re very happy to hear other people’s clever takes on things that are worth doing. It helps us re-examine our thinking.
Here’s the PubMed link again for the “a single low blood pressure” matters paper linked above:
And here’s the one on capnography and major trauma:
The image for this post came from flickr’s Creative Commons area. It is unchanged from the original posting by “Peter”
If you made it this far a reminder that there are options, probably within this very page, to follow along so you get an email when a post turns up.